Our goal is to develop new and effective preventive/therapeutic strategies for various diseases by combining bone biology, oral science and osteoimmunology. To accomplish our goal, we use multi-omics analyses of human samples, bioinformatics, loss-of-function experiments with genetically modified mice, and molecular cell biology techniques. This multidisciplinary approach enables us to model and study the complexity of human diseases.
Our current areas of interest fall under six broadly defined themes below.
1. Anti-tumor effects of non-immune cells
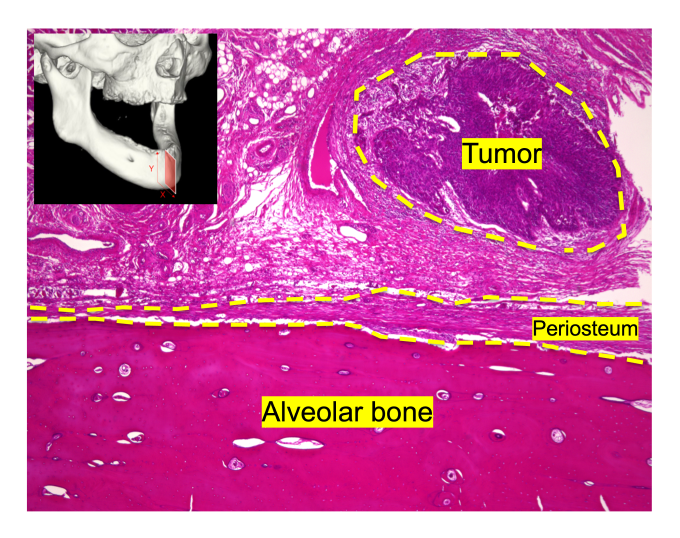
The immune system has a central role in regulating cancer progression, but the anti-tumor effects of non-immune cells remain largely undefined. Recently, we found that stromal cells in the periosteum produce the protease inhibitor Timp1, which inactivates matrix-degrading proteases, promoting periosteal thickening to physically inhibit cancer invasion into the bone. This discovery led us to propose the concept of “stromal defense against cancer: SDAC,” a unique anti-tumor machinery mediated by non-immune stromal cells (Nakamura, Tsukasaki* et al., Nature, 2024). Currently, we are working to explore the roles and mechanisms of SDAC machinery in other cancer types, and to understand detailed molecular mechanisms underlying how non-immune system recognizes the malignant tumors.
2. Arteriosclerosis and vascular calcification
The total number of deaths caused by atherosclerotic cardiovascular diseases is comparable to that of cancer. As atherosclerosis progresses, vascular smooth muscle cells undergo calcification, which significantly worsens clinical outcomes by increasing cardiac load and causing embolism or rupture of blood vessels. However, the detailed molecular mechanisms underlying the progression of atherosclerosis and vascular calcification remain poorly understood, and effective preventive and therapeutic strategies have yet to be established. Given that immune cells infiltrate the arterial wall and local inflammation induces the ectopic calcification, the osteoimmunological perspective would be essential to fully understand the vascular pathogenesis. We are currently investigating the mechanisms of atherosclerosis and vascular calcification from the viewpoint of immune-bone-vascular cross-talks, with the goal of developing novel preventive and therapeutic strategies.
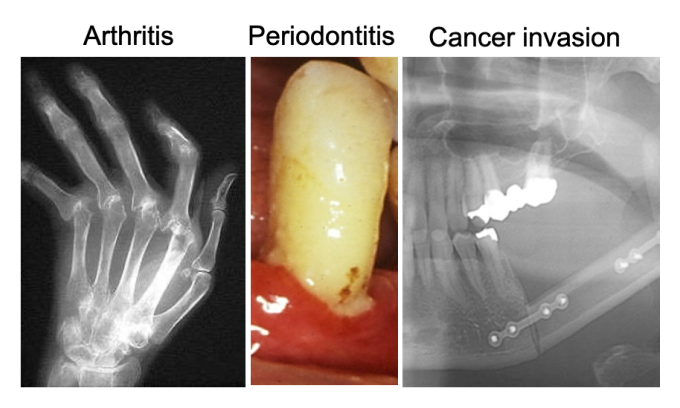
3. Inflammatory bone diseases
The aberrant and/or prolonged activation of immune system causes inflammatory bone destruction. We have been studying the cellular and molecular mechanisms of inflammatory bone damage by focusing on the pathogenesis of periodontal disease, rheumatoid arthritis, and bone invasion/metastasis of cancer(Tsukasaki et al., JBMR 2017, Tsukasaki et al., Nature Communications 2018, Tsukasaki et al., Nature Reviews Immunology 2019, Yan, et al., Nature Immunology 2022, Ando, Tsukasaki* et al., IJOS 2024, Nakamura, Tsukasaki* et al., Nature, 2024)。We are conducting research projects to further elucidate the pathogenesis and to develop therapeutic strategies for tissue damage associated with various pathological conditions, including but not limited to periodontitis, arthritis and cancer.
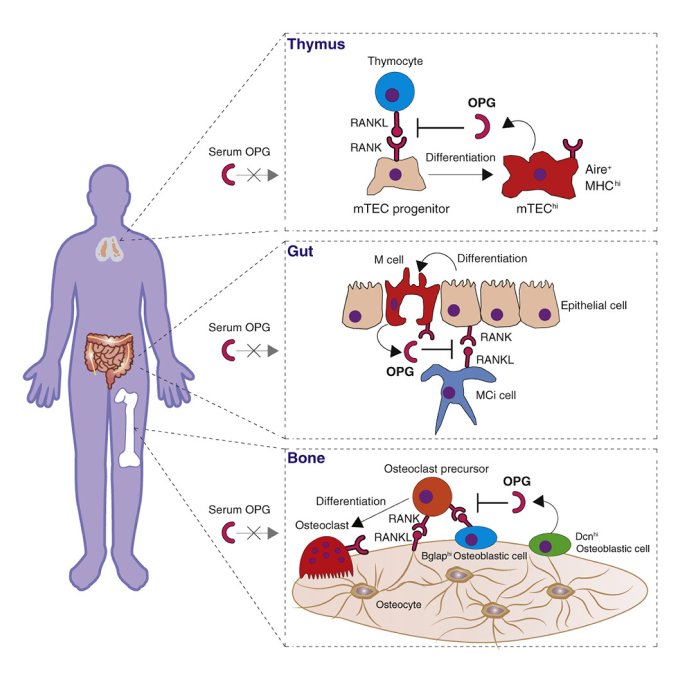
4. RANKL・RANK・OPG story
In 1998, osteoclast differentiation factor RANKL was cloned by this lab led by Prof. Tatsuo Suda, in collaboration with a research team in Snow Brand Milk Products Co., Ltd (Yasuda et al., PNAS 1998). RANKL/RANK/OPG is a central system regulating bone metabolism and also has an essential role in the development and maintenance of immune tissues such as the thymus, lymph nodes, and the intestinal mucosa(Tsukasaki et al., Nature Reviews Immunology 2019). We have identified cell type- and context-dependent regulatory mechanisms of RANKL expression(Yan, et al., Nature Immunology 2022, Yan, Tsukasaki* et al., Bone Research 2023, Ando, Tsukasaki* et al., IJOS 2024). We also developed OPG conditional knockout mouse and specified the cellular sources of OPG in the bone, thymus and gut(Tsukasaki et al., Cell Reports 2017). Currently, we are exploring the role of the RANKL/RANK/OPG system beyond the bone and immune tissues, and investigating the lineage and fate of cells that express RANKL/RANK/OPG by using newly created genetically engineered mice.
5. Skeletal physiology and pathology
The bony skeleton plays essential roles in vertebrate homeostasis by functioning as a locomotor organ, a mineral reservoir, an endocrine organ as well as a primary lymphoid organ. We have been working on osteoclast biology(Tsukasaki et al., Nature Metabolism 2020) and osteoblast/skeletal stem cell biology(Tsukasaki et al., Nature Communications 2022) to provide a comprehensive picture of the skeletal physiology and pathology. Currently, we are conducting several new projects focusing on osteoclast fusion mechanisms, functional diversity of osteoblasts, role of peripheral nerves distributed in the periosteum, and the cross-talks between the skull and brain.
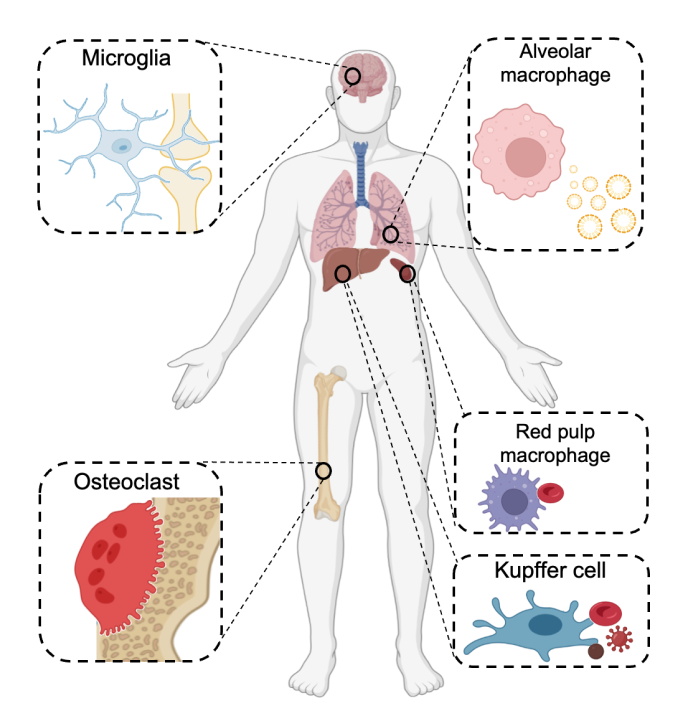
6. Tissue macrophage biology
Tissue macrophages are distributed in nearly the entire body, and contribute to the development and maintenance of various organs. The properties of tissue macrophages are closely linked to the organ-specific functions. Osteoclasts, the tissue macrophage in the skeleton, function as the exclusive bone-resorbing cells by secreting hydrochloric acid and proteolytic enzymes. Brain microglia contribute to the regulation of neuronal metabolism and synaptic connections, and alveolar macrophages maintain lung homeostasis by processing surfactants. Tissue macrophage differentiation and function are thought to be controlled by both a cell-intrinsic transcriptional program and the resident tissue microenvironment, but the detailed mechanisms underlying tissue macrophage regulation remain largely unknown. While exploring regulatory factors of osteoclasts, we identified a novel gene that commonly controls tissue macrophage development. Based on this finding and our expertise of osteoclast biology, we are currently working on multiple types of tissue macrophage to develop therapeutic strategies for various diseases, such as neurological disorders involving microglia and respiratory diseases involving alveolar macrophages.